From the onset of the COVID-19 pandemic, testing has been a critical tool in helping identify cases so COVID-positive individuals can isolate and recover.
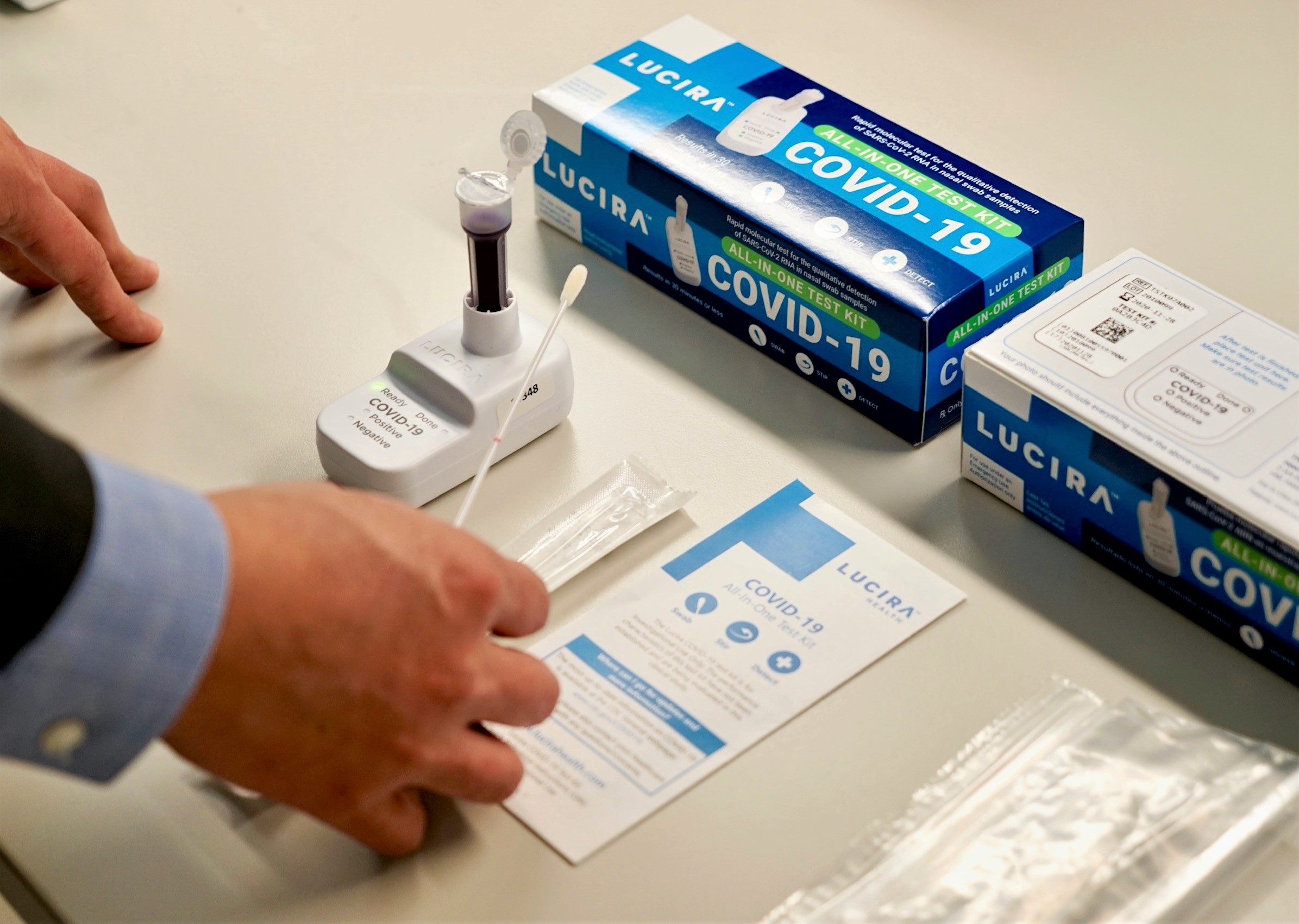
With COVID-19 cases spiking across the country, the need for more rapid and diverse testing options is crucial. Enter the Lucira COVID-19 All-In-One Test Kit. Granted Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) on Nov. 18, this self-administered kit was authorized as the first prescription at-home COVID-19 test for individuals 14 years and older.
“Being able to quickly determine if a person is infected or not has been a global problem,” said John Chou, M.D., medical director for anesthesiology, diagnostics and pharmacy at the Palo Alto Foundation Medical Group and a principal investigator on the Lucira Health Community Testing Study submitted to the FDA. “We believe this highly mobile test can make a big difference by providing lab-quality results expeditiously and conveniently. Early, accurate detection is vital to delivering appropriate care and controlling the pandemic.”

Dr. John Chou, Sutter’s Palo Alto Medical Foundation
How It Works
Lucira’s COVID-19 All-In-One Test Kit is a molecular in vitro diagnostic test that has an analytical sensitivity, or ability to detect the SARS-CoV-2 virus (COVID-19), comparable to molecular tests performed in clinical settings and high complexity labs.
The single-use test kit can produce a positive or negative result within 30 minutes. It has a simple “swab, stir and detect” design. Research revealed that clinical trial participants successfully performed the process in about 2 minutes.
After the test’s completion, patients are instructed to report their results to the medical office or provider who prescribed the test.
Field Tested, FDA-Approved
Sutter’s Palo Alto Medical Foundation launched the study of the Lucira COVID-19 All-In-One Test Kit to diagnose the COVID-19 infection back in July. Sutter Health is now gearing up to offer the test to qualified patients in the next two months.
Currently, Sutter performs an average of 1,600 COVID-19 tests daily in ambulatory settings. Turnaround times for these lab-based diagnostic tests average 24-48 hours.
The Lucira test is expected to be available for providers in the Sutter Health network to prescribe within the next couple of months across the 24 California counties where the health system operates. It will be prescribed for at-home use as well as deployed across the network’s ambulatory settings, such as Respiratory Care Clinics, where patients go for COVID-19 testing.
South Florida’s Cleveland Clinic will be the other U.S. health system to initially offer Lucira’s self-tests to patients. By spring 2021, the all-in-one kit is expected to be available nationally through other healthcare providers.
Vaccines Are Here; Does Testing Still Matter?
Testing, vaccines, contracting tracing and more are all tools in the healthcare toolbox to help contain the virus and reduce its spread. Vaccination at a large scale will take many months into 2021, which means testing will continue to play a crucial role for preventing case spikes as well as additional surges like the one we are seeing now.
“With the introduction of entirely at-home test kits, like the Lucira COVID-19 All-In-One Test Kit, the role of the healthcare provider will become more important, not less,” Dr. Chou, said. “Providers within the Sutter Health network will evaluate patients to determine if they meet FDA guidelines for a test, prescribe the test, review the results with patients, arrange for any follow-up care.”