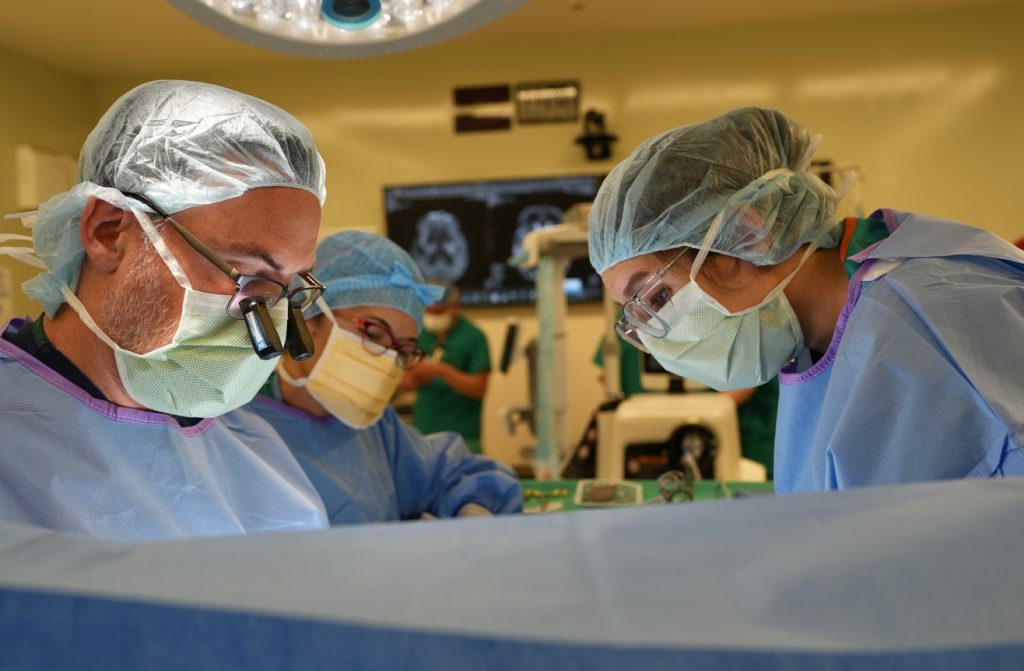
Sutter enrolls first patient in cutting-edge gene therapy clinical trial
What if you couldn’t hug your child, speak a full sentence or lift your morning coffee mug? That’s the reality for thousands living with Amyotrophic Lateral Sclerosis, or ALS, a disease that slowly robs people of their ability to move, speak and breathe. While there is currently no cure for ALS, advances in research hold the potential for new possibilities.
One such effort is underway at Sutter Health’s Forbes Norris MDA/ALS Research and Treatment Center, where doctors recently enrolled the first patient in a Phase I/II clinical trial for AMT-162, a gene therapy candidate targeting a rare, inherited, or genetic, form of ALS caused by a mutation in the superoxide dismutase 1 (SOD1) gene. Sponsored by uniQure biopharma B.V., this proof-of-concept trial is assessing the safety, tolerability and initial signs of efficacy of AMT-162.
“For the first time we’re addressing the root cause of ALS, not just treating the symptoms,” says Dr. Hesham Zakaria, a neurosurgeon at Sutter’s California Pacific Medical Center in San Francisco who performed the procedure with his Sutter team. “This early trial could be a turning point for ALS care, giving patients a chance to fight back and see a brighter future for the management of this disease, which presently has few treatment options.”
What is SOD1: The SOD1 gene provides instructions for making an enzyme called superoxide dismutase, which is abundant in cells throughout the body. This enzyme attaches, or binds, to molecules of copper and zinc to break down toxic, charged oxygen molecules called superoxide radicals. Mutations in the SOD1 gene cause the protein to misfold and clump together. Learn more.
ALS Steals Time. This Trial Aims to Give It Back
ALS causes motor neurons in the brain and spinal cord to break down, leaving muscles too weak to function and eventually resulting in paralysis. For those with the SOD1 mutation – a small subset of ALS patients – nerve damage progresses even faster, in months instead of years, with an average survival of about 2.7 years.
According to the ALS Association, half of all people affected by ALS live at least three years after diagnosis. Twenty percent are likely to live five years or more, and up to 10% will live more than 10 years. Most notably, famed theoretical physicist Stephen Hawking lived 55 years with the disease, though he had a different, more slowly progressive form, and his case was considered an anomaly.
Unlike Hawking, people with the SOD1 mutation face a more aggressive form of the disease, where nerve damage accelerates rapidly. AMT-162 takes a unique approach to treatment. Delivered via intrathecal administration, the gene therapy is introduced into the spinal fluid, allowing it to travel directly to the nervous system. By reaching the nervous system, AMT-162 targets the root cause of the disease and aims to suppress – or ‘knock down’ – the harmful protein that drives disease progression. “The goal is to stop the damage at its source,” explains Dr. Zakaria.
Dr. Zakaria’s counterpart, Dr. Jonathan Katz, director of the Forbes Norris Center, says, “This is more than research. It’s hope. We’re pushing into a new frontier of ALS care, and the courage of the first patient to take this investigational therapy is helping lead the way for thousands of others.”
A Community Hospital Helping Lead the Charge
For over 40 years, Sutter Health’s Forbes Norris Research and Treatment Center has been a national leader in ALS care.
According to Dr. Katz, what makes this research milestone even more remarkable is that Sutter’s CPMC is the only non-university hospital involved in the trial thus far. Dr. Katz says, “This proves that cutting-edge treatments don’t just belong at big academic centers. They can and should be available to patients everywhere, right in their communities.”
As of January 2025, CPMC is one of only four centers in the nation participating in this Phase I/II trial.
Do you or a loved one have ALS? Find help here and explore research and clinical trials at Sutter.