According to the Alzheimer’s Association, about one in nine Americans aged 65 or older has Alzheimer’s disease. By 2050, these numbers are projected to more than double, growing to an estimated 12.7 million people across the United States.
As cases rise, researchers worldwide continue searching for factors influencing how the disease starts and progresses.
Charting a DNA Roadmap
A new, first-in-kind study at Sutter Health aims to help answer key questions that define how the illness can best be diagnosed, treated and potentially even prevented.
This research project is sponsored by Kuakini Medical Center in Honolulu, Hawaii through a grant awarded by the National Institute on Aging with Sutter Health, Massachusetts General Hospital and the University of Hawaii as collaborating institutions
Led at Sutter by Dr. Gregory Tranah, senior scientist at Sutter’s Sequoia Center for the Science of Aging, the study team is taking a novel approach to mapping DNA mutations across the brain.

Dr. Gregory Tranah
“We’re looking at small alterations in the DNA inside brain cells that may be associated with Alzheimer’s,” explains Dr. Tranah. “A handful of these changes that are acquired over a lifetime may increase disease risk, while others may protect people. By examining these patterns, we aim to identify why some individuals develop Alzheimer’s and others are resilient to the disease, so treatments may be started earlier in people at higher risk.”
Data-Driven Discovery
To make this study possible, the research team is utilizing data from one of the world’s largest and most comprehensive aging studies, sponsored by Kuakini Medical Center through its Kuakini Honolulu Heart Program and Kuakini Honolulu-Asia Aging Study.
Since 1965, this research has followed thousands of American men of Japanese ancestry, collecting detailed health, memory and biological data and specimens.
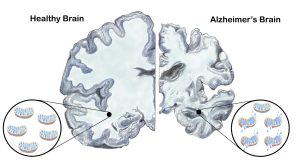
Mitochondria play multiple critical roles including providing cellular energy, regulating apoptosis, and maintaining oxidative balance. Mitochondrial dysfunction in the brain is a hallmark of Alzheimer’s Disease. Illustration by Shana Katzman
This new collaboration enables Dr. Tranah’s team to examine frozen brain and blood samples from 400+ Kuakini Honolulu Heart Program and Kuakini Honolulu-Asia Aging Study participants. The team will look at DNA changes across six different parts of the brain and study whether the same signals appear in blood, and how they connect to long-term memory and brain functioning.
“This unique resource allows us to build a brain mutation map tied to more than 50 years of patient data,” says Dr. Tranah. “It presents an opportunity to witness DNA changes alongside actual memory test results.”
Mitochondria: The Brain’s Hidden Power Source
At the core of the study is mitochondrial DNA. Known as the powerhouse of cells, mtDNA provides energy and keeps the brain and other organs functioning smoothly.
“As we age, mtDNA is more likely to develop small changes,” says Dr. Tranah. “When measured in a lab, these tiny changes can impact how the brain cells work, offering a chance to spot the disease quicker.”
The study also aims to discover if these DNA changes in the brain can be detected in blood.
The research team at Massachusetts General Hospital in Boston is studying blood samples from Kuakini Honolulu Heart Program and Kuakini Honolulu-Asia Aging Study participants to identify signals for Alzheimer’s disease.
“Developing a blood-based screening test to catch these changes before symptoms occur may help doctors detect the disease earlier, which would be game-changing for millions of patients,” says Dr. Tranah. “We are coming together to create a brain ‘atlas’ that may pave the way to a brighter future of Alzheimer’s disease care.”
Translating Science into Hope

Dr. Shawn Kile
“Alzheimer’s is a much-too-common and devastating disease, but we have entered a new era in which there is hope,” says Dr. Shawn Kile, chair of Sutter’s Neuroscience Service Line. “This study is important because we can hope to better detect the earliest indications of pathology present at the start of this disease. By expanding our capabilities for early identification and intervention, we will have the best chance of slowing down or even preventing dementia, potentially even prior to the first symptoms of memory loss.”
“This research is ultimately about giving answers to patients and families,” says Dr. Tranah.
Do you or a loved one have Alzheimer’s disease? Find advanced neuroscience services and learn more about research and clinical trials at Sutter Health.