Few hospitals in the U.S. have the necessary equipment and expertise to treat large vessel occlusion (LVO) ischemic stroke. This challenges emergency medical services staff who must quickly and accurately identify stroke type to ensure individuals are transported to a facility with the appropriate level of care.
A new, non-invasive wearable headset is helping care teams identify LVO strokes in the field, with Sutter Health participating in this pioneering research.
“A key challenge in stroke care is the lack of tools to detect stroke type in the pre-hospital setting before an individual arrives at the emergency room,” says Dr. Nobl Barazangi, Sutter West Bay Medical Group neurologist and medical director of stroke and neurocritical care research for the Sutter Bay Region. “At Sutter, we’re proud to offer patients access to stroke centers equipped to treat even the most severe types of strokes with research helping pave the way for advances in clinical care.”

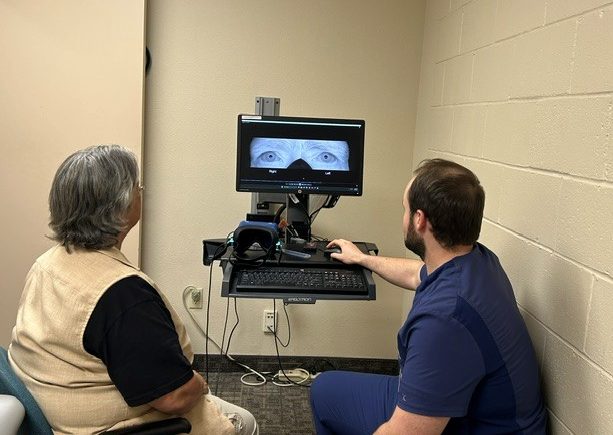
MindRhythm proprietary headset embedded with HeadPulse technology
With stroke, EMS crews do not have objective measures at their fingertips, such as electrocardiogram, to accurately identify the stroke’s condition. Although pre-hospital assessments such as the Cincinnati Prehospital Stroke Scale (CPSS) and the Los Angeles Motor Scale (LAMS) are helpful, studies have shown these assessments are only about 60% accurate.
Now, research findings from the pilot study EPISODE-PS-COVID suggest a specialized headset called Harmony may significantly improve the detection and assessment of LVOs. This means stroke specialists can administer effective treatment earlier, says Dr. Barazangi.The Harmony headset, developed by MindRhythm, leverages a measurement technique called accelerometry. Accelerometers measure motion and acceleration or vibrations. They are widely used across a range of technologies, including smartphones to track motions like shaking, tilting and rotating. Similarly, the Harmony headset uses accelerometry to collect a signal that detects slight motion of the brain after each heartbeat. Disruption in this signal, called HeadPulse, from a normal pattern indicates damage to the brain. In stroke, this damage is caused by a clot interfering with normal blood flow to the brain.
Promising Study Findings
EPISODE-PS-COVID clinical trial results showed the Harmony device has a sensitivity and specificity that is non inferior, or not worse, to the LAMS score when tested in individuals suspected to have experienced a stroke and whose type of stroke is unclear.
In addition to analyzing sensitivity and specificity compared to LAMS, research data was collected from EMS teams to test the headset design for optimal device interaction and function. The EPISODE study is the largest pre-hospital stroke device trial of its kind. A significant percentage of the study population was Black males, a high-risk population for stroke often underrepresented in clinical research.
In October 2023, the specialized headset received Breakthrough Designation from the U.S. Food and Drug Administration. The designation was granted to help assess suspected stroke patients in the pre-hospital setting and to determine whether the patient may have a LVO stroke.Sutter Health was one of a few study sites in Northern California that participated in EPISODE-PS-COVID. Findings were published in the Journal of Stroke Vascular and Interventional Neurology.
Sutter is participating in the next phase of this research, the EPISODE_VS study whose findings will be used for submission to the FDA next year.