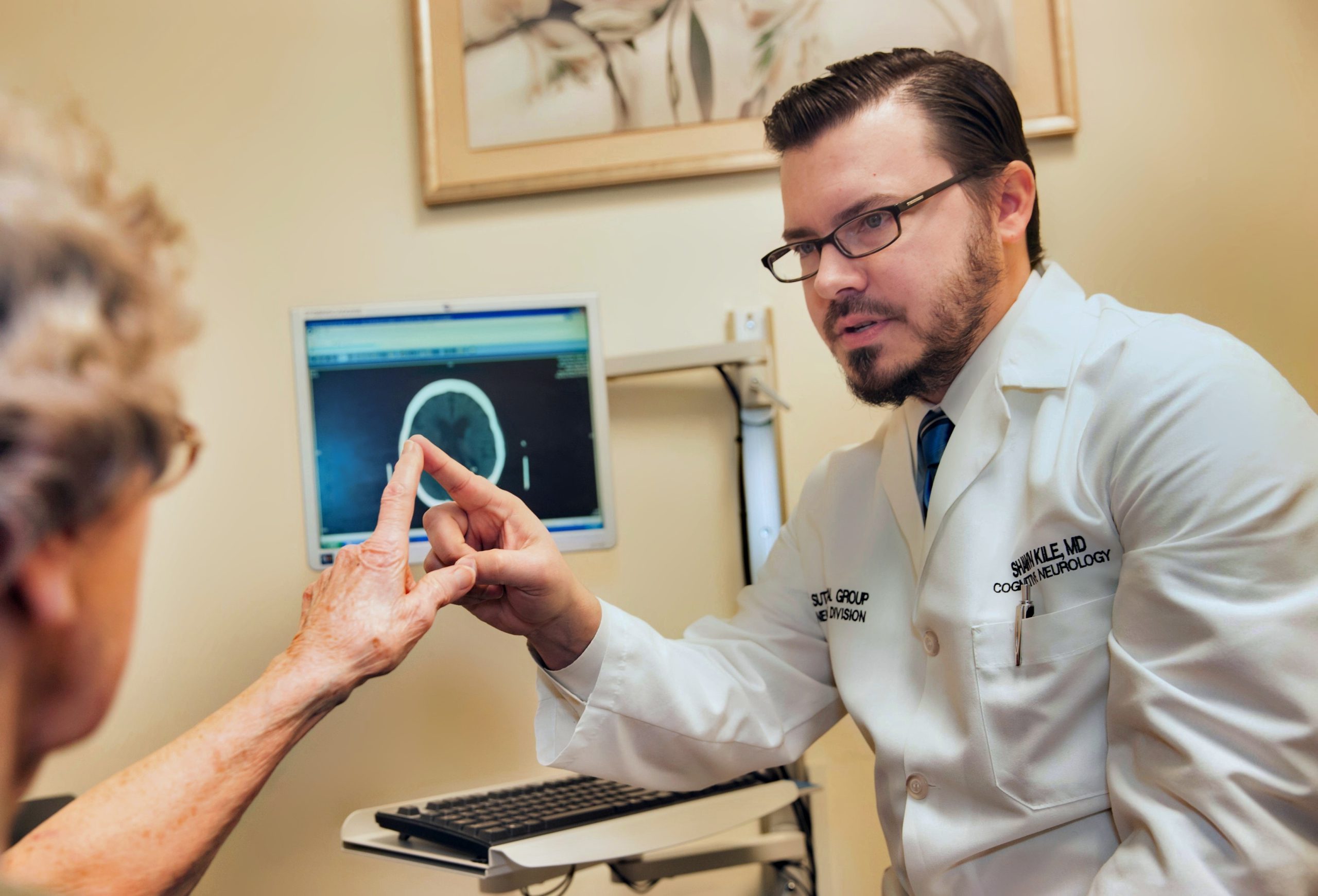
Dr. Shawn Kile, medical director of the Sutter Neuroscience Institute, is the founder of the groundbreaking Sutter Memory Clinic in Sacramento. He is a principal investigator for clinical trials of new therapies for Alzheimer’s disease and adult autism.
By Marika Rose, Vitals contributor
In early 2021, Claudia Shelly began to lose track of which day of the week or date it was. When her forgetfulness and difficulty with day-to-day planning became more pronounced, she was naturally concerned that other factors were at play beyond the usual effects of aging.
Claudia’s late mother’s decline from Alzheimer’s disease was dramatic and devastating, and she was concerned she may also be facing the same illness. “I noticed that mentally things weren’t lining up for me, and I was getting really frustrated,” recalls Claudia, a 72-year-old resident of Sacramento, Calif.
In March 2023, she was referred to Dr. Shawn Kile, a neurologist with Sutter Health in Sacramento, who evaluated Claudia’s cognitive (brain) health and functioning. He performed a neurological assessment that indicated patterns consistent with the early signs of Alzheimer’s.
Claudia underwent MRI brain imaging and a cerebral spinal fluid testing commonly known as a spinal tap to determine if her brain biomarkers confirmed findings of the initial memory assessment.
A new era in the treatment of Alzheimer’s disease
At the time, Dr. Kile was aware of clinical trial data for a new Alzheimer’s treatment called lecanemab (Leqembi™) — later approved by the U.S. Food and Drug Administration in July 2023 as the first treatment to affect the underlying biology of the disease in individuals with early-stage Alzheimer’s.
 In a large clinical study, after 18 months of treatment, lecanemab slowed the rate of cognitive decline by about 27% in patients with early-stage Alzheimer’s symptoms including mild cognitive impairment, which is a pre-dementia stage as in Claudia’s case, or mild dementia.
In a large clinical study, after 18 months of treatment, lecanemab slowed the rate of cognitive decline by about 27% in patients with early-stage Alzheimer’s symptoms including mild cognitive impairment, which is a pre-dementia stage as in Claudia’s case, or mild dementia.
Unlike other currently approved treatments for the Alzheimer’s disease, lecanemab uses an immune strategy to remove beta-amyloid plaques that accumulate in the brain in patients with Alzheimer’s. Lecanemab is given by IV (intravenous) infusion every two weeks, with each infusion lasting about one hour.
“This is an exciting time in neurology with new treatments for individuals with Alzheimer’s disease,” says Dr. Kile. “This new generation of treatments — lecanemab being the first fully approved by the FDA — shows promise by actually clearing out amyloid pathology in Alzheimer’s disease. If this treatment is initiated early enough, prior to significant neurodegeneration, then there is real chance that we can impact the rate of disease progression.”
Need-to-know about this new treatment
Dr. Kile says that, while this new drug is promising, patients need to be carefully selected and closely monitored during the course of treatment. The benefits and risks should also be weighed carefully.
As with all drugs, lecanemab can have side effects. Some individuals receiving treatment may experience side effects which can include dizziness, headache, confusion, as well as brain swelling or bleeding.
 Not all individuals may benefit from treatment with lecanemab: the drug is not approved for individuals without any signs of cognitive decline or those in the later stages of Alzheimer’s disease. People who have a gene called ApoE ε4 are at increased risk of brain bleeding or swelling, especially if they carry two copies of this gene.
Not all individuals may benefit from treatment with lecanemab: the drug is not approved for individuals without any signs of cognitive decline or those in the later stages of Alzheimer’s disease. People who have a gene called ApoE ε4 are at increased risk of brain bleeding or swelling, especially if they carry two copies of this gene.
To be considered for lecanemab treatments, individuals should typically be 50 to 90 years of age with mild cognitive impairment or mild dementia, have MRI completed within one year before starting treatment, and agree to additional monitoring MRIs during treatment, undergo a PET scan or lumbar puncture testing to identify biomarkers for Alzheimer’s, and agree to enroll in an Alzheimer’s disease registry.
Although there is little evidence that lecanemab can reverse the decline already incurred by Alzheimer’s disease, Shelly reported improvement in mental clarity and executive functioning.
“My hope with lecanemab is that I outlive this disease — that when it’s my time it won’t be due to the progression of Alzheimer’s,” added Claudia. “After seeing my mother suffer as her disease progressed, my husband and I are grateful to have this opportunity and are now hopeful that we will continue to fully enjoy our life together and time with our family for years to come.”
Click here to find neuroscience services offered at Sutter Health.