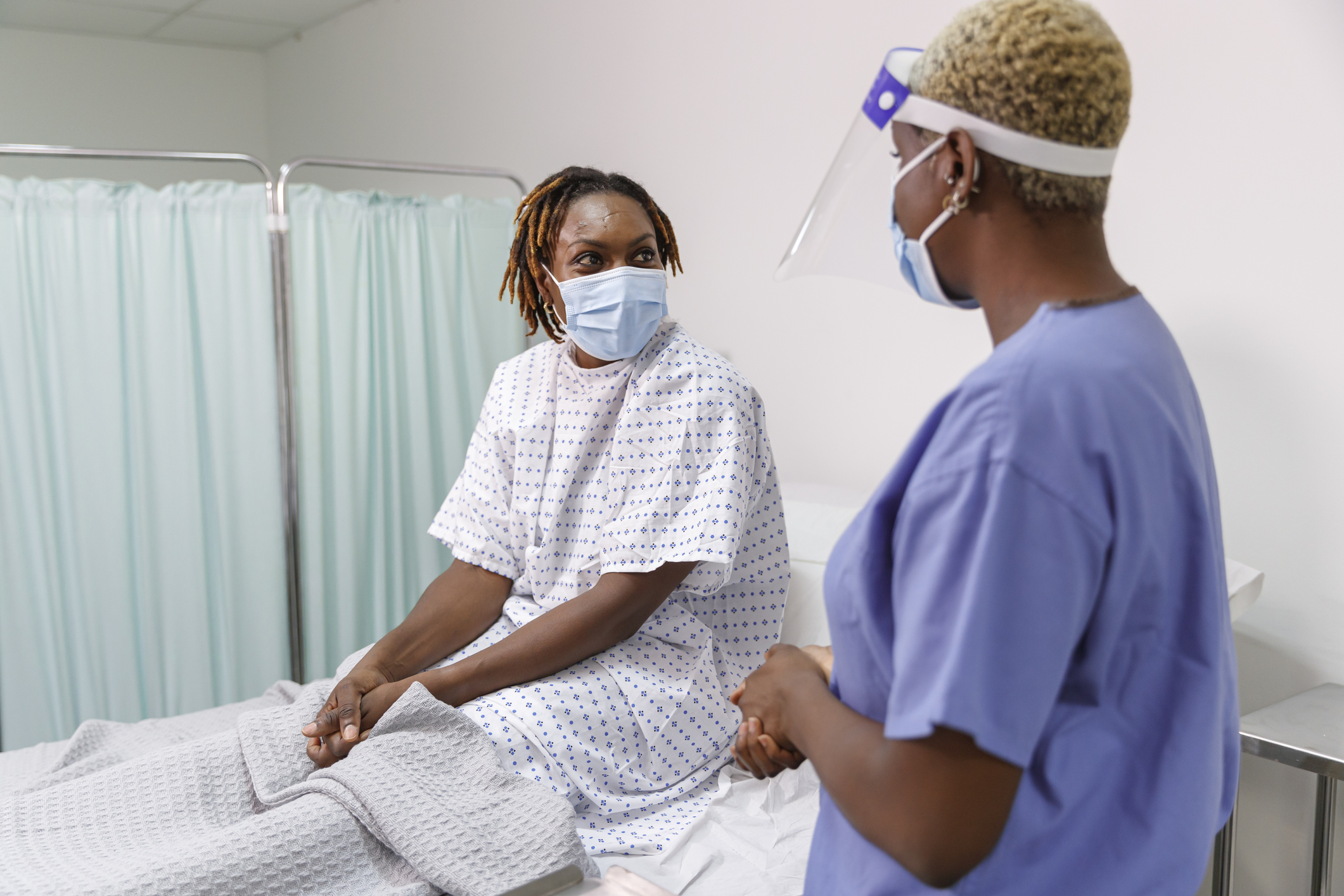
Clinical trials are the very foundation of novel drug and device discoveries. They also have a complicated history of racial, social and economic inequities, as illustrated most recently by research stating Black women with metastatic breast cancer do not have the same access to clinical trials. Healthcare researchers state what’s needed now is a framework from which to build greater equity. Doing so will help ensure that every person has a fair and just opportunity to achieve equitable outcomes.
In a newly published article in the Journal of Clinical Pathways, Kristen M.J. Azar, scientific medical director for Sutter Health’s Institute of Advancing Health Equity (IAHE), and Dr. Alice Pressman, IAHE research director emeritus, lay out five critical strategies for enhancing clinical trial diversity. Both researchers acknowledge that achieving results will require simultaneous collaboration with the FDA, health delivery systems, like Sutter Health; research funding organizations; public and private agencies and foundations; and pharmaceutical and medical device companies.
“To achieve equity in health outcomes, we must also achieve equity in clinical research,” said Azar. “It is our collective responsibility to come together and develop solutions that foster a more equitable system.”