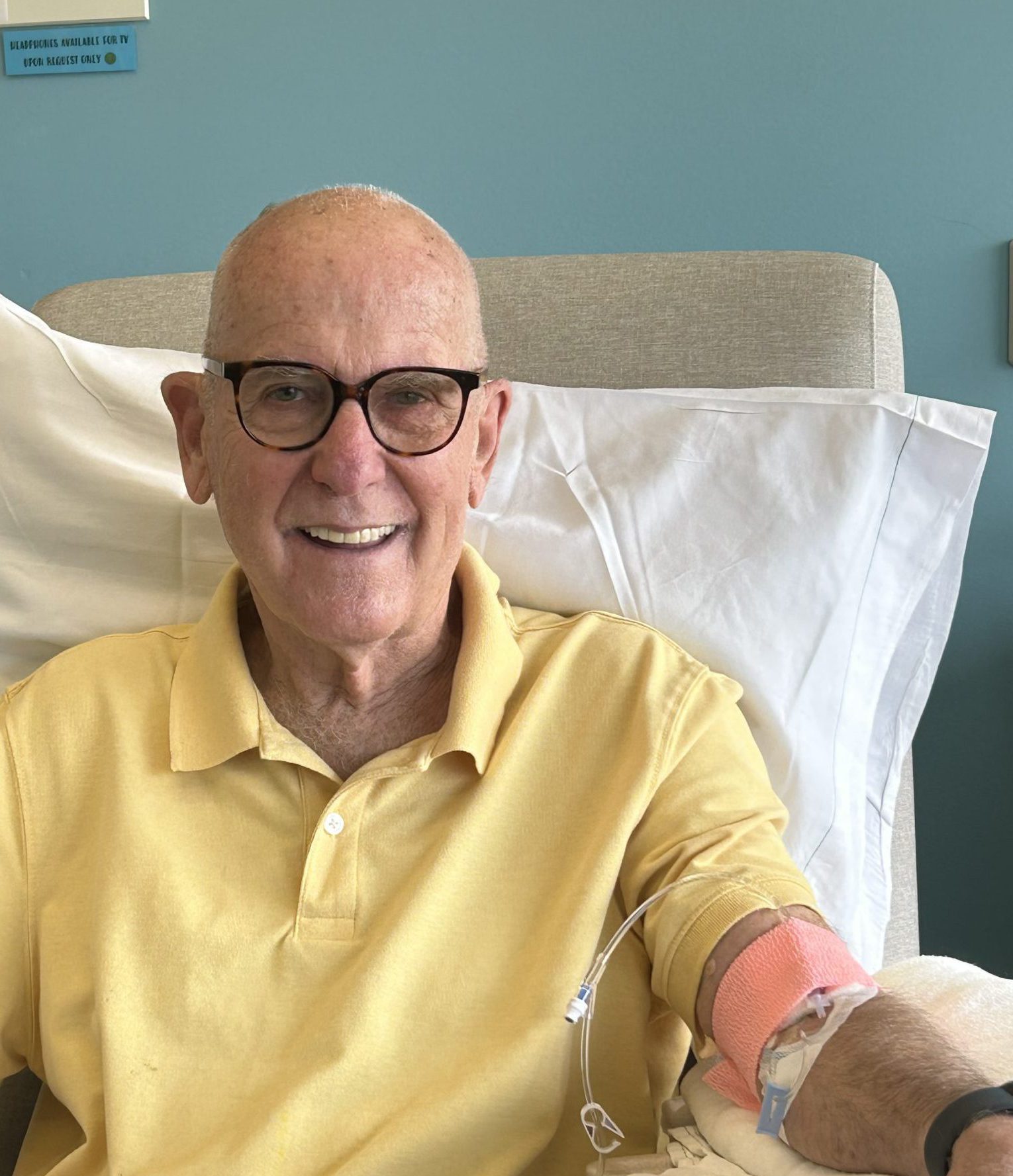
(Above) Pat Ross receiving an infusion of the Alzheimer’s disease drug lecanemab.
Pat Ross calls it his “happiness regimen”: An antioxidant-rich breakfast, exercise at the local YMCA and time spent enjoying artwork or volunteering. He follows the routine daily, not for the health and wellness benefits it may bring but for the joy it adds to his life.
“Happy-go-lucky is the only way I know how to be,” says the retired 74-year-old Santa Rosa, Calif., resident and grandfather of five kids.
Ross is one of the hundreds of patients receiving care for Alzheimer’s disease, dementia and other memory disorders across Sutter Health’s integrated network of community hospitals. And although the course and speed of his disease progression may be uncertain, advances made possible by research, shared learning among experts, and an approach at Sutter to whole-person health are bringing hope so happiness may not be forgotten.
In 2022, worsening forgetfulness prompted Ross to seek medical advice from his primary care provider. Referred to neurologists at Sutter Santa Rosa Regional Hospital and Sutter’s California Pacific Medical Center in San Francisco, MRI-based 3D imaging of Ross’s brain revealed abnormalities concerning for potential signs of Alzheimer’s disease.
A neurological assessment and cerebral spinal fluid testing confirmed patterns and markers consistent with mild cognitive impairment (MCI) due to Alzheimer’s disease. MCI is the earliest symptomatic stage of Alzheimer’s disease.
Alzheimer’s Care for the Body, Mind and Spirit
“The risk of Alzheimer’s disease increases with age, so as people live longer, more and more are developing this often-devastating illness that affects the entire family. At Sutter Health we’re bolstering our efforts to bring holistic, personalized care to individuals with cognitive impairment and their families, delivering new science and solutions to approach the disease from all angles,” says Dr. Armen Moughamian, section chief of memory for Sutter’s neuroscience service line and medical director of the Ray Dolby Brain Health Center at CPMC—one of the leading centers of its kind for research and clinical expertise to care for individuals with Alzheimer’s disease, dementia and other memory disorders.
Born in the Bay Area, Dr. Moughamian trained in neurology and research at the University of California, San Francisco. “I feel strongly connected to the San Francisco Bay Area community and I am passionate about providing the best evidence-based care for people suffering from dementia and degenerative diseases of the brain,” he says.
It’s that depth of commitment and passion that individuals feel and see from Dr. Moughamian and the care team comprised of nurses, neuropsychologists, and social workers at the Ray Dolby Brain Health Center. The center expanded last year to give more patients access to its groundbreaking treatment programs and will see its services amplified in the new Sutter Advanced Neuroscience Complex at Mission Neighborhood, which will open to patients in 2028.
Patients’ caregivers experience the benefits, too: Ross’s oldest daughter Lindsay says, “To see my dad cared for with capable hands and compassionate hearts brings such relief and trust to me, my siblings and our families. We don’t know how fast or aggressively dad’s disease may advance, but we do know we have the right resources in place to preserve quality of life with his care established at Sutter.”
That bond of trust is one Dr. Moughamian and colleagues safeguard while continuing to reinforce the infrastructure of support offered at the Ray Dolby Brain Health Center.
“We continually enhance our educational materials and support resources for patients and their caregivers, while making them easier to access right where patients live,” says Dr. Moughamian. “We are deeply grateful for the partnership and philanthropic support from the Dolby Family which allows us to provide this highest quality of care.”
He emphasizes Sutter’s efforts to raise awareness among the general public and family physicians of the importance of early recognition of Alzheimer’s signs and symptoms, to help aid prompt diagnosis and treatment.
Ushering in New Treatments
“This is an exciting time in neurology with new treatments for individuals with Alzheimer’s disease,” says Dr. Moughamian. “A new generation of drugs approved by the U.S. Food and Drug Administration – lecanemab in July 2023 and donanemab one year later – show significant promise by engaging the immune system to remove beta-amyloid plaques that accumulate in the brains of patients with Alzheimer’s. If these treatments are started early enough before significant neurodegeneration, we can slow the rate of disease progression and keep patients in these earlier stages longer.”
At Sutter Santa Rosa, Ross receives bimonthly intravenous infusions of lecanemab (Leqembi™). The drug was FDA-approved as the first treatment to slow the rate of cognitive decline in individuals with early-stage Alzheimer’s symptoms including MCI or mild dementia. During telehealth video visits, Dr. Moughamian meets with Ross and his family to monitor his experience with the drug and watch for any potential side effects or signs of complications.
“Right from my first visit with Dr. Moughamian, and before I started taking lecanemab, he garnered my trust and confidence. I would have said yes to Tootsie Rolls if he offered them as treatment,” recalls Ross with a chuckle. “It eases my mind and comforts my family to know my in-person care in San Francisco is closely coordinated with video visits and the treatment I receive at Sutter’s infusion therapy center in Santa Rosa.”
For Dr. Moughamian, lecanemab and other disease-modifying, monoclonal antibody drugs mark a pivotal shift toward a brighter future for Alzheimer’s disease treatment.
But while lecanemab and other new Alzheimer’s therapies are promising, Dr. Moughamian says patients need to be thoughtfully selected and closely monitored during the course of treatment. The benefits and risks should also be weighed carefully.
He and other Alzheimer’s disease specialists at Sutter meet weekly to review the medical charts of patients who may benefit from the treatment to ensure appropriate patient selection and sharing clinical best practices to help ensure patients have the ideal outcomes. The goal of this review board is to improve safe access to these novel treatments throughout the Sutter Health system.
Dr. Shawn Kile, a neurologist, medical director of the Sutter Neuroscience Institute and chair of Sutter’s neuroscience service line founded the groundbreaking Sutter Memory Clinic at Sutter Medical Center, Sacramento. He’s also Dr. Moughamian’s colleague, collaborator and “sounding board” as they brainstorm and make possible new solutions for better and more coordinated Alzheimer’s disease care.
“Disease-modifying drugs that slow the rate of Alzheimer’s disease represent one of most exciting advances we’ve seen in decades in this field of research and medicine,” says Dr. Kile. “But like other highly sophisticated drugs that are administered by infusion, it takes a closely connected team of multidisciplinary experts collaborating to safely bring it to patients when and where they need it.”
Dr. Kile credits the teamwork and expertise of physicians, specialists, infusion therapy nurses and pharmacists across Sutter who quickly assembled a team upon FDA approval of lecanemab to “put patients first,” enabling the drug and more like it to be offered to eligible patients like Ross across the Sutter system.
“If it wasn’t for Sutter, I wouldn’t be able to enjoy all the people, places and experiences that are making me happy,” says Ross.
Novel Treatment Option
Based on findings from the TRAILBLAZER-ALZ2 clinical trial, the FDA approved donanemab (Kisunla™) July 2, 2024, for the treatment of adults with MCI or mild dementia stages of Alzheimer’s disease.
Sutter is participating in the TRAILBLAZER-ALZ3 and TRAILRUNNER-ALZ3 clinical trials that aim to prevent or delay cognitive decline before it starts.
“Amyloid plaques are the first changes that occur in Alzheimer’s disease. However, as Alzheimer’s disease advances, there is less contribution of amyloid to disease progression and other factors such as tau that drive the disease. These pre-symptomatic studies aim to remove amyloid plaque as early as possible—prior to the onset of memory loss symptoms—and hopefully will significantly delay the onset of cognitive changes. This is very similar to treatment of breast cancer using a mammogram instead of waiting for a woman to feel a mass before the initiation of diagnosis and treatment,” says Dr. Moughamian.
“I am extremely excited about these prevention trials for Alzheimer’s disease, proud of Sutter’s participation in the studies and deeply grateful to the patients in our network who join clinical trials to help champion the importance and impact of research. If we can delay disease onset, we will dramatically improve quality of life for our patients,” says Dr. Moughamian.
Alzheimer’s: Not a Normal Part of Aging
7.2 million Americans have Alzheimer’s disease, and the number is projected to reach 13.8 million by 2050. Most people develop the disease in their 70s and 80s, and it is the most common cause of dementia in older adults.
Alzheimer’s disease is irreversible. It’s a progressive neurodegenerative disorder that slowly erodes an individual’s cognition, memory and brain function. Individuals with severe cases of the illness may lose the ability to communicate, independently perform activities of daily living and even walk or swallow.
Do you or a loved one have a memory disorder like Alzheimer’s disease or dementia? Find more information about Sutter CPMC’s Ray Dolby Brain Health Center and the Sutter Memory Clinic at Sutter Medical Center, Sacramento.