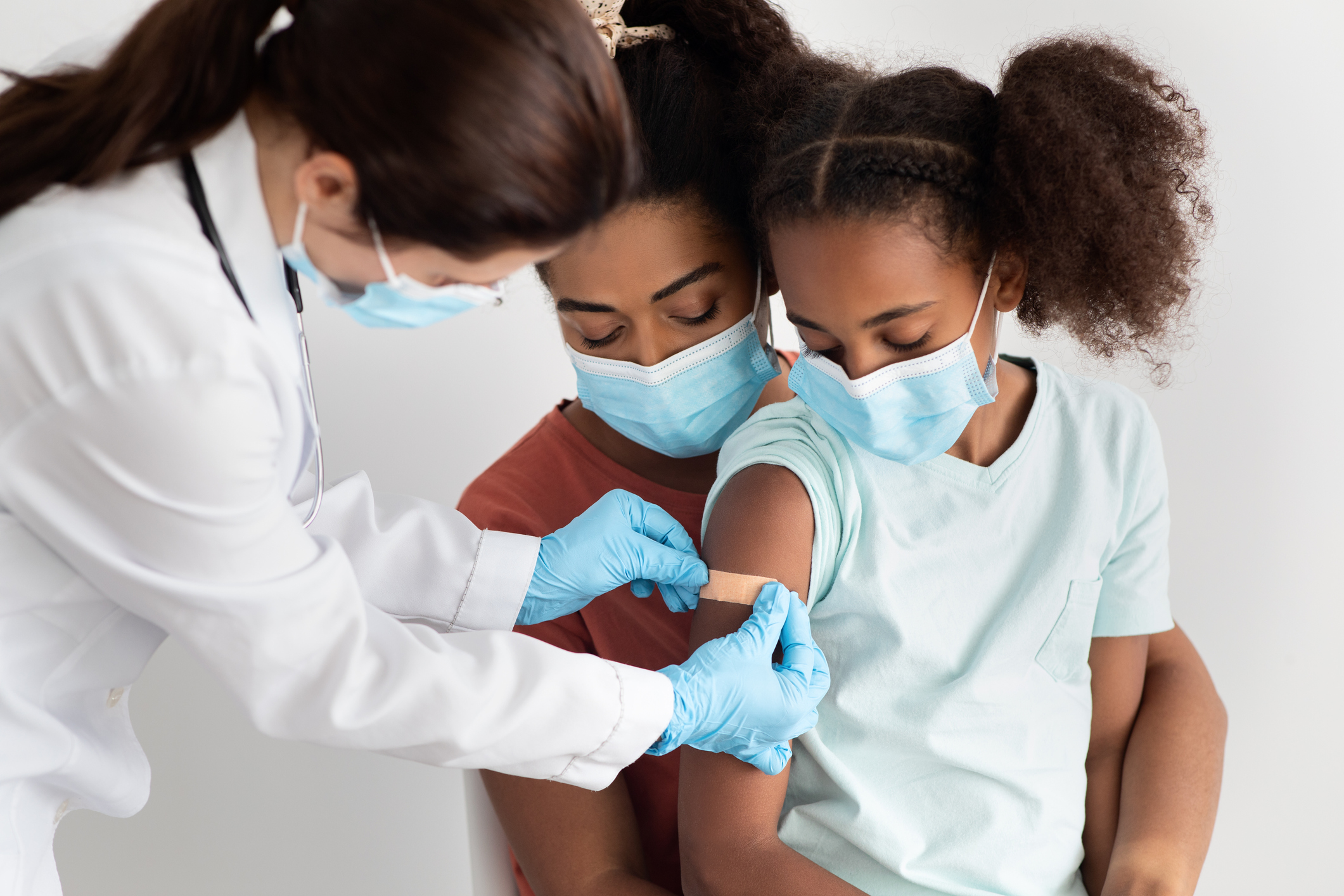
FDA approval, as well as other regulatory guidance, has cleared a path for the administration of Pfizer’s COVID-19 vaccine for children 5 to 11. Naturally, parents and other caregivers have lots of questions. Vitals tackle some of the top inquiries.
Is the vaccine safe for children 5 to 11 years old?
As stated by the Centers for Disease Control and Prevention, scientists conducted clinical trials before recommending COVID-19 vaccination for children. Based on the research, analysis and expert discussions, the Food and Drug Administration gave the Pfizer-BioNTech COVID-19 emergency authorization to use in children ages 5 to 15 years old. Previously the FDA had granted full approval to use in young people ages 16 years and older.
The CDC states that COVID-19 vaccines are continually monitored for safety with the most rigorous and detailed monitoring program in U.S. history. While there can be risks with any vaccination, the CDC says serious health events are rare post-COVID-19 vaccination. Parents and guardians can learn more about the development and approval process for COVID-19 vaccinations via the CDC.
When and where can I make an appointment to vaccinate my child?
In anticipation of the high demand, parents have several options for pediatric COVID-19 vaccinations. Opportunities range from retail pharmacies and various community clinics, including those within area schools and through local departments of public health. Additionally, various healthcare systems are available to support vaccinations.
The CDC suggests that children ages 5 years and older receive a COVID-19 vaccine for as soon as possible. Parents and guardians may choose to take the first available appointment for their child.
What is unique about the vaccine for kids versus adults?
While those 12 years and older receive the same dosage of Pfizer-BioNTech COVID-19 vaccine as adults, the CDC states children ages 5 to 11 require their own age-appropriate dose. Unlike other medications, COVID-19 vaccine dosage does not vary by patient weight. Rather it is determined by age on the day of vaccination. The Pfizer-BioNTech COVID-19 vaccine dose for 5- to 11-year-olds is just one-third of the adult dose, according to the CDC. Additionally, the CDC states that smaller needles for the Pfizer-BioNTech COVID-19 vaccine will be used for those 5 to 11 years old.
There is one similarity between adult and pediatric Pfizer-BioNTech COVID-19 vaccines: they require two doses. According to the CDC, children 5 to 11 years old will need a second shot of the Pfizer-BioNTech COVID-19 vaccine three weeks after their first dose.
More helpful information and resources for parents and caregivers is available via the CDC on their dedicated COVID-19 Vaccines for Children and Teens page.
In anticipation of the high demand, parents have several options for pediatric COVID-19 vaccinations. Opportunities range from retail pharmacies and various community clinics, including those within area schools and through local departments of public health. Additionally, various health care systems, including Sutter Health, are available to support vaccinations.