Happenstance can take many shapes. But for 46-year-old Sutter patient Vashti Ross, who was found to have a rare genetic mutation that caused Amyotrophic Lateral Sclerosis (or “ALS”, sometimes called Lou Gehrig’s disease), the diagnosis provided her the unique opportunity to enroll in VALOR, a Phase 3 clinical trial of a new treatment for ALS that was approved by the U.S. Food and Drug Administration (FDA) last month.
Diagnosed with ALS at age 37, Vashti first sought medical advice from a neurologist in Portland, Ore., where she lives. With no family history and only a slight “hitch” in her step, the diagnosis was shocking.

Vashti Ross
“I began searching for news of drugs or other therapies that might help slow the progression of my disease,” says Vashti. But her primary care doctor and neurologist counselled Vashti that ALS remains an illness without a cure.
With only a handful of potential therapies in the research pipeline that might target Vashti’s specific genetic mutation – called SOD1 – Vashti’s physicians advised that a clinical trial might be the best way to slow the progression of her ALS.
What is ALS?
ALS is a rare, progressive neurodegenerative disease that affects motor neuron nerve cells in the brain and spinal cord. When the motor neurons die, the brain’s ability to start and control muscle movement is lost, causing symptoms such as muscle twitching and weakness in a limb or slurred speech. Eventually, ALS affects control of the muscles needed to move, speak, eat and breathe.
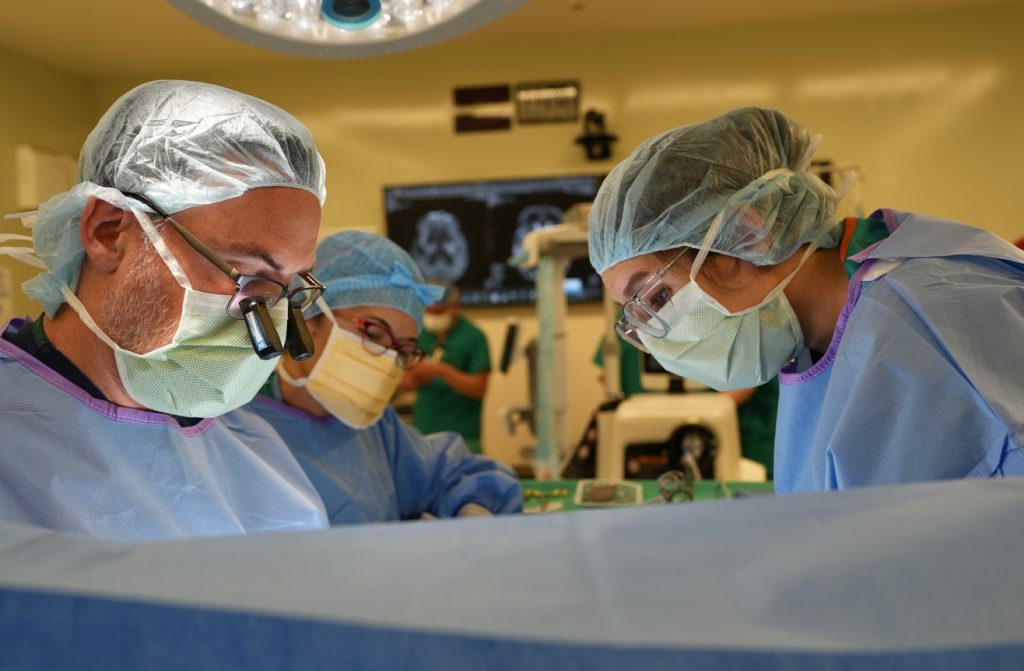
Vashti’s neurologist in Portland searched for clinical trials and found one that matched Vashti’s specific type of the disease. The catch: the clinical trial was being offered at Sutter’s California Pacific Medical Center (CPMC) in San Francisco, at the nationally renowned Forbes Norris MDA/ALS Research and Treatment Center. CPMC was the only site in Northern California and the north-western U.S. offering enrollment to the study.
Vashti made a quick decision: the travel would be worth the opportunity to participate in the clinical trial testing a new, investigational drug that might slow the progression of her illness and ease her symptoms.
“It was a privilege to be a part of this study,” says Vashti. “Research brings the medical field understanding, and understanding brings us closer to a cure. I hope patients who benefited from this study will participate in further studies. It was extremely satisfying to learn that my participation contributed to this new therapy.”
She joined the VALOR clinical trial in 2019 and traveled to San Francisco each month to receive treatment with Qalsody™ (tofersen), a medication made by the pharmaceutical company Biogen.
“The most pressing challenge of ALS is finding a treatment that is effective in slowing the progression of disease. Despite decades of scientific research, there are still only few medications approved by the U.S. FDA to treat ALS, and some have limited effectiveness in halting the persistent degeneration of motor nerves in the brain and spinal cord associated with the illness,” says Dr. Liberty Jenkins, Sutter neurologist and director of the ALS Clinic at the Forbes Norris Center.
Today, other clinical trials are underway for individuals who are at risk of developing ALS because they carry the SOD1 gene mutation but who have not yet shown symptoms.
“Starting treatment before nerve damage is clinically detectable may help prevent the development of ALS,” says Dr. Jenkins. “My hope is that research and clinical trials at Sutter Health can improve outcomes not only for patients like Vashti who have been diagnosed with a genetic form of ALS, but also individuals who have sporadic ALS, where no particular cause has been identified,” she adds.
To learn more about research and clinical trials at Sutter Health, contact ClinicalResearch@sutterhealth.org.